White House releases new #COVID19 preparedness plan

I'm not going to mince words here: While the Omicron variant wave of the COVID pandemic appears to have mostly subsided nationally (the 7-day new case average has plummeted from an all-time high of around 800,000/day nationally in mid-January to around 55,000/day now), I think the seemingly across-the-board abandonment of mask mandates at the federal, state and local levels is still a big mistake.
I would have waited until new daily cases drop further (to perhaps 10 per 100,000 per day, or around ~33,000/day nationally) and hold at that rate or lower for a solid month before giving the "all clear" for vaccinated folks to remove their masks at most indoor settings.
For unvaccinated people, of course, I'd want them to be required to wear masks indoors in public until they actually get vaccinated (which, aside from young children, nearly all of them should have done already; it's been nearly a year since they've been widely available, for God's sake).
(If that means anti-vaxxers have to wear masks indoors the rest of their lives, so be it, though obviously that's not going to happen.)
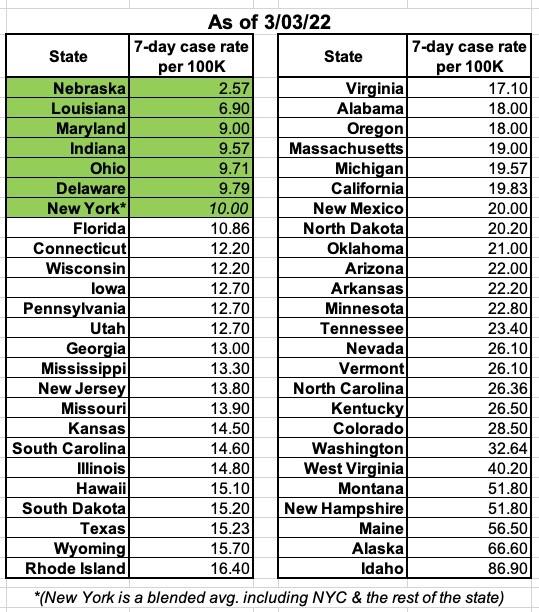
Of course, there are parts of the country which have dropped to that average case rate (Nebraska, Louisiana, Maryland, Indiana, Ohio, Delaware and New York are all at or below 10/100K as of this writing), but none of them have stayed that low for very long.
For what it's worth, here's where each U.S. state stands on this measure as of today (in some cases the most recent 7-day average data is a few days old):
All of this being said, the bottom line is that for good or for bad, most of the country seems to have decided to mostly demask. Instead, during his State of the Uniont speech a few days ago, President Biden announced several new COVID initiatives...and the White House website has posted more details on its new "National COVID-19 Preparedness Plan" which does include some important new measures:
Today, the U.S. government is releasing the National COVID-19 Preparedness Plan – which will enable America to move forward safely, sustaining and building on the progress we’ve made over the past 13 months. This plan lays out the roadmap to help us fight COVID-19 in the future as we begin to get back to our more normal routines. We look to a future when Americans no longer fear lockdowns, shutdowns, and our kids not going to school. It’s a future when the country relies on the powerful layers of protection we have built and invests in the next generation of tools to stay ahead of this virus.
The President’s National COVID-19 Preparedness Plan focuses on four key goals:
Protect Against and Treat Covid-19
The Administration will work with Congress to secure the necessary funding to:
- Launch an effort to vaccinate America’s youngest children as soon as the U.S. Food and Drug Administration (FDA) authorizes and the CDC recommends a vaccine for that age group.
If the FDA authorizes and CDC recommends a vaccine for children . If the FDA authorizes and the CDC recommends a vaccine for children under five years of age, the United States is prepared to immediately distribute vaccines through a network of thousands of pediatricians’ offices, children’s hospitals, health centers, and local sites, so that vaccines are made available conveniently to families across the country.
Ensure that Americans – of all ages – can get the protection of an effective vaccine.
To ensure that people stay protected, the U.S. government will The Administration will continue to ensure that all Americans have ready access to free and safe vaccines, because vaccines are the most effective defense against COVID-19. The U.S. Department of Health and Human Services (HHS) will also continue to monitor the efficacy and durability of currently authorized vaccines against current and future variants and make recommendations to optimize protection.
Increase American manufacturing capacity to reliably produce an additional 1 billion vaccine doses per year – three times the U.S. population – and accelerate research and development of a single COVID vaccine that protects against SARS-CoV-2 and all its variants, as well as previous SARS-origin viruses.
To ensure that people stay protected, the U.S. government will continue to use advance purchasing agreements when appropriate and work closely with vaccine manufacturers to produce shots quickly and safely. Fully supporting this effort to scale up domestic vaccine manufacturing will require additional resources from Congress. Additionally, we will maintain a network of tens of thousands of sites to deliver shots to the American people at any time this effort is needed.
Continue vaccination outreach and education efforts and combat misinformation and disinformation.
HHS will continue its work to equip Americans with the tools to identify misinformation and to invest in longer-term efforts to build resilience against health misinformation.
Ensure there are enough treatments for every American who needs them.
The U.S. government will procure additional treatments; continue to use an expedited, streamlined process to review treatments for authorization by the FDA; and accelerate research and development into next generation treatments. These efforts will require additional funding and authorities from Congress.
Launch a nationwide Test to Treat Initiative so Americans can rapidly access treatment, including by visiting a “one-stop” location to get a free test and free treatment pills.
The Administration will put forth new educational efforts for the public and providers so that Americans can rapidly access treatments. The Administration will establish “One-Stop Test to Treat” locations at pharmacy-based clinics, community health centers, Long-Term Care Facilities, and the U.S. Department of Veterans Affairs (VA) facilities across the country. “One-stop” sites will be operational by March.
This "Test to Treat" program is potentially a big deal/game changer...assuming it's implemented properly and equitably, and assuming these new COVID treatments from Pfizer and other drugmakers are as effective as I've been hearing (it's my understanding that they're only effective when given very early on in the infection process, however). And (as noted below) this still won't solve a very real problem for the immunocompromised community.
Update the framework for recommendations on preventive measures like masking to reflect the current state of the disease.
Masks have been a critical tool to protect ourselves, but they have a time and a place. With a broad range of other protective tools in place, the CDC has announced an updated framework for guidance on preventive measures like masking – moving away from simply basing broad recommendations on case counts and test positivity, and instead encouraging prevention measures like masking when they are most needed to minimize severe disease and to keep our hospitals from becoming overwhelmed in times when COVID-19 is surging. By monitoring community risk, masks can be worn when the risk of severe disease in the community is high and taken off when the risk is low. Overall, it means Americans will be wearing masks less because so many people are protected from severe disease.
(Again, I agree that there will come a point when mask mandates are no longer necessary in indoor settings, I just disagree that we've reached that point in most areas of the country yet.)
- Launch a one-stop-shop website that allows Americans to easily find public health guidance based on the COVID-19 risk in their local area and access tools to protect themselves.
The Administration will launch a website where Americans can find the level of COVID-19 risk in their community and specific guidance based on that risk. The site will also point people to the tools we now have to fight COVID-19, such as locating a vaccination site in their neighborhood or finding a free high-quality mask at a local grocery store or pharmacy.
Honestly I kind of thought that either the CDC.gov or Vaccines.gov pretty much served this purpose already, but whatever.
Sustain and increase American manufacturing of COVID-19 tests, so we can continue to have a robust supply of tests.
The Administration will continue to use the expedited authorization process to help test manufacturers get tests to market quickly; maintain America’s network of thousands of free testing sites; use the Defense Production Act (DPA) and other authorities, where warranted, to increase manufacturing capacity; and invest in innovation to make tests less expensive. These continued investments in testing will require additional funding from Congress.
Prioritize protections for the immunocompromised and take new actions to protect people with disabilities and older adults.
The Administration will continue to provide strong support for the immunocompromised, including providing prioritized access to treatments and preventive interventions – pending additional funding from Congress – as well as ensuring access to boosters. The Administration will also increase equitable access to testing and COVID-19 mitigation resources for people with disabilities and older adults, and engage industry to accelerate research and development of accessible self-tests. Securing sufficient preventive treatments for people who are immunocompromised will require additional funding from Congress.
This is the issue/policy which I've the most disappointed about, and it's the main reason I think abandonment of most mask mandates is still premature. The disability community, particularly those who are immunocompromised (tens of millions of Americans) is understandably still extremely concerned about being exposed to a deadly, highly contagious pathogen which many of them don't have much immunity to even when fully vaccinated & boosted.
I'm glad that their concern merited its own bullet point, but I'm pretty sure they'd prefer more support for mask mandates for awhile longer yet.
Help Americans with the long-term impacts of COVID-19.
In recognition of the wide-reaching long-term impacts of COVID-19 on our society, the President will direct the U.S. government to accelerate efforts to detect, prevent, and treat Long COVID; coordinate efforts to provide support to families who have experienced the COVID-related loss of a loved one; and attend to the mental health and well-being of our communities. The Administration will also propose to make new investments in health care workers to support their mental health and well-being.
Ensure equitable access to COVID-19 health care and public health resources.
The Administration will continue to prioritize providing equitable access to COVID-19 health care and public health resources – including personal protective equipment (PPE), tests, treatments, masks, and vaccines; and address COVID-related health inequities among communities defined by race, ethnicity, geography, disability, sexual orientation, gender identity, and other factors. The U.S. government will support dedicated resources for local community-based organizations, community health centers, and rural health clinics.
Prepare for New Variants
The Administration will work with Congress to secure the necessary funding to:
Improve our data collection, sequencing, and wastewater surveillance capabilities to immediately identify and detect new and emerging variants; and strengthen pandemic preparedness.
The U.S. government will continue improvements to COVID-19 The U.S. government will continue improvements to COVID-19 disease and vaccination data collection, wastewater surveillance, and virus sequencing capacity so we are better prepared to respond rapidly to emerging threats. This includes strengthening data infrastructure and interoperability so that more jurisdictions can link case surveillance and hospital data to vaccine data. The Administration is also leveraging COVID-19 response capabilities into stronger pandemic preparedness.
The 'wastewater surveillance' move is an important one. As gross as the issue may be, the bottom line is that viruses (not just COVID but other types) can often be detected early on via the raw sewage collected at wastewater treament facilities. This is a leading indicator, so if there's a surge in COVID there, it likely means that there will be a surge in cases and illness in that community shortly after that.
Leverage a COVID-19 Variant Playbook to determine the impact of a new variant on our vaccines, treatments, and tests, and shore up and update our tools, if needed.
The Administration has developed a variant playbook to rapidly assess the disease severity and transmissibility of a new variant, and to expedite the rapid laboratory evaluation of the effectiveness of vaccines, tests, and treatments against any variant. The U.S. The Administration has developed a variant playbook to assess the disease severity and transmissibility of a new variant immediately, and to expedite the rapid laboratory evaluation of the effectiveness of vaccines, tests, and treatments against any variant. The U.S. government has also developed a series of plans in coordination with manufacturers for the accelerated development, approval, manufacturing, and delivery of updated vaccines, tests, and treatments. These expedited plans and processes suggest that updated vaccines can be deployed in 100 days instead of many months or years.
Utilize new FDA processes to expedite regulatory review of variant-specific versions of vaccines and treatments, so America can get them in place, if needed.
FDA has developed new processes to accelerate the authorization and approval of a vaccine or treatment that targets any new variant while maintaining strict and longstanding practices to ensure the safety and efficacy of the products.
Support new FDA processes to expedite regulatory review of variant-specific versions of vaccines and treatments, so Americans can get them quickly if needed.
FDA has developed new approaches to accelerate the authorization of a vaccine or treatment that targets any new variant while maintaining strict and longstanding practices to ensure the safety and efficacy of the products.
Leverage a proven COVID-19 Surge Response Playbook.
The Administration has developed a comprehensive emergency response COVID-19 surge playbook to stand up mass vaccination and testing sites, expedite deployments of surge medical and emergency personnel, expand hospitals and emergency facilities, and provide emergency supplies.
Add at-home tests, antiviral pills, and masks for the general population to America’s stockpile for the first time.
America will stockpile new categories of supplies including at-home tests, antiviral pills, and masks for the general population for the first time. The Administration will also maintain a fully stocked Strategic National Stockpile (SNS) with an inventory of masks, ventilators, gloves, gowns, and hospital equipment. The U.S. government will be ready to deploy supplies to the American people to ensure adequate supply in times of surges, COVID-19 outbreaks, or new variants.
The U.S. government has established a permanent logistics and operational hub at HHS to ensure accelerated development, production, and delivery of COVID-19 vaccines and treatments.
The Administration has transitioned an emergency logistics and operational organization into a permanent agency structure at HHS, which has allowed the Administration to build on its progress, retain expertise and skills, and continue providing the necessary tools to the American people during this pandemic and for any future disease outbreaks.
Prevent Economic and Educational Shutdowns
The Administration will work with Congress to secure the necessary funding to:
Give schools and businesses guidance, tests, and supplies to stay open, including tools to improve ventilation and air filtration.
The U.S. government will also provide a Clean Air in Buildings Checklist that all buildings can use to improve indoor ventilation and air filtration and will encourage uptake of ventilation improvements. The Administration will also provide technical assistance that encourages schools, public buildings, and state, local, and Tribal governments to make ventilation improvements and upgrades using American Rescue Plan funds.
Work with Congress to provide paid sick leave to workers who need to miss work due to a case of COVID-19 or to care for a loved one who has COVID-19.
The Administration will work with Congress to reinstate tax credits to help small- and mid-size businesses provide paid sick and family leave to deal with COVID-related absences.
Update guidance for employers to ensure safer workplaces.
The Department of Labor’s Occupational Safety and Health Administration (OSHA) will update workplace guidance to better equip employers with the tools they need to ensure safe workplaces, including guidance on how employers can continue to support increased vaccination and boosting of their employees; support workers such as people who are immunocompromised who choose to wear high-quality masks; limit workplace-based infections; and enhance ventilation.
Engage early care and education providers to help them remain safely open and help parents return to work with peace of mind.
Early care and education providers, including child care centers, family child care providers, pre-K and more, have been essential in our fight against COVID-19. The Administration invested $40 billion in American Rescue Plan funds to states, territories, and Tribes to help child care providers and Head Start grantees keep their doors open and provide safe care that is crucial for parents getting back to work. Building on this funding, the Administration will continue to engage the community of early care and education providers to ensure they have tools and support to stay safely open and to continue supporting our families.
With the vast majority of federal workers at their workplaces, substantially expand levels of services at public-facing federal offices (like local Social Security offices).
COVID-19 no longer needs to dictate how we work. Federal agencies will lead by example, increasing the hours public-facing federal offices are open for in-person appointments and in-person interactions in the month of April.
Continue to Lead the Effort to Vaccinate the World and Save Lives
The Administration will work with Congress to secure the necessary funding to:
Leverage the vaccine donation model America pioneered to deliver the 1.2 billion doses we committed to donate to the rest of the world.
America will continue to deliver the 1.2 billion doses we committed to donate to countries in need, continuing to leverage the partnerships the U.S. government built to donate and deliver vaccines to the rest of the world.
Increase efforts to get shots in arms around the world.
The U.S. government will increase investment in the Initiative for Global Vaccine Access (Global VAX), an ambitious global vaccination initiative to get doses into arms by working with partner countries to more quickly implement their plans. This includes supporting efforts such as jumpstarting communications campaigns, providing and supporting vaccinators on the front lines, purchasing cold chain supplies and syringes, paying for shipping and logistics to expedite vaccine delivery to hard-to-reach areas, ensuring people at high risk of hospitalization and deaths like the elderly and immunocompromised are vaccinated, and building vaccine confidence on the ground. Expanded global shots-in-arms efforts will require additional funding from Congress.
Save lives by solving the oxygen crisis and making emergency supplies widely available.
The U.S. government will make oxygen and PPE available; enhance testing; provide treatments; strengthen global health systems to fight COVID-19; protect health workers from COVID-19 and essential health services from COVID-19 disruptions; improve detection, monitoring and mitigation of new COVID-19 variants; and increase regional and local manufacturing of countermeasures. These continued investments will require additional funding from Congress.
Continue global leadership on the COVID-19 response and build better health security for the future.
The U.S. government will continue to work to build better capacity to fight COVID-19, manage future variants, and advance health security and preparedness for future pandemics. America is committed to establishing a new health security financial intermediary fund at the World Bank in 2022, and we call on all countries and public and private organizations to commit to urgent action to assist in the global COVID-19 response.